We Are Jumo Health: Ryan Mercer
An interview with graphic designer, Ryan Mercer
Great companies are made up of great people. Here at Jumo Health, our most valuable resource is our team. We are a collective of medical folk, product people, designers, and storytellers that share a common goal to change health care today. Through our We Are Jumo Health series, we will introduce you to the dedicated people who are the heartbeat of Jumo Health.
This month, we’d like you to meet Ryan Mercer, who has been a graphic designer at Jumo Health since July 2020. Ryan lives in Wilson, NC with his wife and three children.
Why did you join Jumo Health?
I was introduced to Jumo through my sister-in-law (Ashley McGee) when she hopped on board a few years ago. She had just traveled to NY to meet everyone for the first time and was telling us about it at our family Christmas party. She glowed when she told us about the culture at Jumo—the shared passion to make health literacy more accessible. (I’ve heard the scholarly term for this is Collective IQ.) Though I didn’t have a background in healthcare, her excitement was infectious, and when an opportunity to join the design team opened up several months later, I jumped at the chance.

Describe a day in the life of your job.
One of the most exciting things about working at Jumo is the variety of products we offer. I could be working on the layout for a study’s website, tweaking comic book text, and designing logo concepts all in the same day. Each product is important and helps tell the patient a story. Either of the study they may be joining, the condition they suffer from, or maybe even the medicine they’ll be taking. No one that I’ve worked with, from client services to editorial to design takes this lightly. Everyone is passionate about delivering the best possible work to tell the best possible story. As a design team, we meet several times a week via Zoom to go over work, collaborate, and just get to know each other. Joe Brady’s done such a great job at creating an environment for us designers to thrive in.

What motivates you?
When we sit down to design something, we try to keep in mind the end user experience. Who will be reading this and how can we make this easy to understand and visually interesting for people from a wide range of backgrounds and cultures? It’s a powerful thing to know that what we create can help affect people’s medical decisions.
Specifically, my faith in Jesus is what motivates me. He’s the ultimate Healer, and I feel honored to be a small part of so many people’s healing journeys.
What are some hobbies or interests you have outside of work?
My family is my world! My wife, Brittney, and I have three awesome kiddos, Hannah (3), Micah (2), and a little girl due in late July. Life is fast and fun in the Mercer house! We do everything together and I wouldn’t trade it for anything.
Besides that, I’m pretty obsessed with typefaces, coffee, sports, and old Star Wars novels (typical nerdy, graphic designer-y things).
Jumo Health’s CEO Invited to Speak about Diversity, Equity and Inclusion in Health Care at the National Action Network 2022 Convention
NEW YORK, NY (April 4, 2022) PRNewswire – Kevin Aniskovich, President and Chief Executive Officer of Jumo Health, will speak at the National Action Network’s 2022 Convention on Friday, April 8 at 4:45pm.
Aniskovich will participate in a panel discussion titled “Health and Wellness in the Wake of COVID.” He will join health care leaders including:
- Dr. Virginia Caine, Director and Chief Medical Officer, Marion County Public Health Department
- Dr. Randall Brown, MD, MPH, Global Lead and Senior Director, Teva Pharmaceuticals
- Rev. Dr. Que English, Director, Center for Faith-Based and Neighborhood Partnerships, U.S. Department of Health and Human Services
- Debra Fraser-Howze, Founder, Choose Healthy Life
- Deborah Wafer, HIV Prevention Medical Scientist, Gilead Sciences
- Tony R. Wafford, President and CEO, I Choose Life Foundation
- Rev. Oliver E. Buie, Vice President of National Action Network Los Angeles Chapter (Moderator)
“Given our focus on diversity, equity, and inclusion, it is an honor to be included on this panel of health care leaders. Jumo Health stands with the National Action Network in its pursuit of one standard of justice, decency and equal opportunities for all people regardless of race, religion, ethnicity, citizenship, criminal record, economic status, gender, gender expression, or sexuality,” shared Aniskovich. “Inequities in health care continue to plague our nation. During this panel, I’ll discuss how our multicultural health literacy initiatives can close these gaps.”
The National Action Network Convention will take place April 6-9, 2022 at the Sheraton New York Times Square Hotel. The purpose of the event is to bring together some of the country’s most influential figures – from top government officials, to faith and civil rights leaders to grassroots organizations. In its 31st year, the event will address our nation’s crisis in racial justice and meaningful forms of action.
“We are so honored to have such a prestigious group of health providers, community outreach organizers, and faith leaders to participate in what we believe will be the beginning of a movement to address health inequities among those most vulnerable,” said Rev. DeVess Toon, National Field Director of the National Action Network.
About National Action Network
National Action Network is one of the leading civil rights organizations in the Nation with chapters throughout the entire United States. Founded in 1991 by Reverend Al Sharpton, NAN works within the spirit and tradition of Dr. Martin Luther King, Jr. to promote a modern civil rights agenda that includes the fight for one standard of justice, decency and equal opportunities for all people regardless of race, religion, ethnicity, citizenship, criminal record, economic status, gender, gender expression, or sexuality. To learn more about NAN, visit https://nationalactionnetwork.net/
About Jumo Health
Jumo Health develops age-appropriate, culturally sensitive, and relatable educational resources for patients and caregivers. We have experience serving diverse populations, covering more than 160 health topics across 75+ countries and 90+ languages – and we’re always expanding! Our multicultural offerings are designed to explain the latest in evidence-based literature using highly visual elements so that everyone can understand complex medical topics. We use familiar mediums to ensure this – from comic books and Pixar®-style animation, to virtual reality experiences and authentic documentary-style patient stories – all tailored based on age and audience. Jumo Health collaborates globally with more than 180 advocacy groups and community organizations to ensure an authentic patient experience is accurately represented. To learn more about Jumo Health, please visit JumoHealth.com.
Enabling the Informed Individual to Make the Right Care Decisions
Health care is complex and can be overwhelming – and even frightening – for many. This is especially true if there are language or cultural barriers to accessing care. Yet understanding care options and care instructions are critical to achieving desired outcomes. Similarly, historical biases and mistreatment of under-represented populations deter many from participation in clinical trials even though broad-based and diverse enrollment is critical to drug development. Global medical communications company Jumo Health has identified a common issue: deficiencies in health literacy and reading comprehension are exacerbating the social determinants of health.
The Jumo Health Approach
Despite clinical and technical advancements, health care still requires the expertise of trained professionals and the exercise of medical judgement. Jumo Health bridges the patient and provider gap by developing age-appropriate and culturally competent materials to address specific conditions, issues, and populations in ways they can understand, relate to, and act upon. The Company has extensive work experience across medical topics, leveraging its deep relationships with patient advocacy and community organizations to ensure its work is based on the most current literature and is peer-reviewed by leading specialists.
Much of Jumo Health’s philosophy dates back to its roots in providing pediatric resources to help children understand their diagnosis and care. Using a combination of authentic storytelling and comic books, Jumo Health started by allowing real patient experiences to come to life on the pages of their comic books. The Company’s focus on authentic and relatable storytelling that is centered on addressing health literacy, reading comprehension, age, and culture has allowed it to expand its solution set and the markets it serves.
Diversifying its Footprint
The Company’s experience serving a pediatric audience gives it a barometer for how to explain the complex in relatable ways to ensure the desired action. Borrowing from that experience, Jumo Health successfully expanded its offerings from condition-specific pediatric resources into the global clinical trials market with a mix of print and digital resources serving all ages across all trial types in more than 70 countries covering more than 80 languages within 150 medical topics.
Jumo Health entered the clinical trial market to cure the historical deficiencies pharmaceutical companies have experienced attempting to enroll participants (80% of trials are delayed due to recruitment issues) and as they struggle to address high dropout rates (nearly half of participants drop out before completion).
Today, the Company creates resources to support recruitment and retention efforts for its pharmaceutical and clinical research organization (CRO) customers. “Central to our clinical trial product suite is a broad range of specific interventions delivered at key inflection points throughout the patient journey,” shared Kevin Aniskovich, the Company’s Chief Executive Officer. “Working with our customers, we [Jumo Health] provide the right resources at the right time to create a roadmap for success. The delivery of these resources mirror the way people consume information, varies by demographic profile, and contemplates condition and study protocol,” continued Aniskovich.
COVID Shines a Spotlight on Diversity
In the wake of COVID-19, drug development, and the process for recruitment of participants in particular, has been in the spotlight. From vaccine hesitancy to the hunt for therapeutics to help the world “return to normal,” Jumo Health stepped in working on various studies related to COVID-19 treatment for sponsors and the US Government.
In addition to the historical deficiencies in clinical trials, those statistics are exacerbated by the discrimination and mistreatment of minority groups and COVID-19 shined a clear and bright light on this topic. The legacy of the Tuskegee trials, the Havasupai Diabetes Project, and the treatment of Henrietta Lacks have all led to historical distrust in the process. In fact, recent studies show that only 15% of African Americans and 6% of Latinx Americans participate in clinical trials. In the Company’s effort to ensure equity in the process, they collaborated with Klick Health and the ACTG for the ACTIV-2 trial, dubbed Rise Above COVID, and earned the Gold Medal in the most recent MM&M Awards for their use of clinical trial marketing and multicultural resources.
The 3 Ms
In developing solutions, Jumo Health applies the 3 Ms to its educational resources – message, medium, and market. In addition to custom materials, a unique feature of Jumo Health is its proprietary library of materials available for license. Noted Aniskovich: “Our library allows study teams to stand up IRB-approved resources fast which is particularly important in rescue trials or for budget-sensitive studies.”
Continued Aniskovich, “Jumo Health’s strength lies in our ability to marry the clinical with the creative in an appropriate way for any target audience.” In fact, since opening its doors, Jumo Health has been delivering some of the most out-of-the-box solutions that redefine the way people look at healthcare. From comic books to VR, Jumo Health offers solutions to support all aspects of health care – from trials to point of care.
Behind the Scenes
Jumo Health’s team works directly with sponsors and CROs of all sizes and with varying budgets. Depending on the project, resources developed may include print and digital resources for patients, or even investigator resources to ensure the message is properly communicated in a relatable way so that patients see themselves as part of the process and understand their role in their care. In addition, Jumo Health offers a broad range of specific interventions at crucial inflection points throughout the patient journey. Aniskovich says, “Our delivery methods mirror the way people consume information and vary based on demographic profile, condition and study protocol. For example, for a pharmacokinetic (PK) study or other trials with long IV sessions, we may recommend a VR experience as a distraction method. This is especially helpful for children, one of the age cohorts that gravitate to video over text.”
In all cases, Jumo Health’s process is based in trust and relies on peer-reviewed, evidence-based content. For each engagement, Jumo Health deploys cross-functional teams representing clinical, product and creative, client services, and advocacy/community to ensure that the final product includes trusted, relevant, and actionable messaging. “Our approach to content creation includes collaborating with advocacy and community, use of storytelling, and peer review. This rigorous process results in a halo of community credibility and a higher level of adoption,” said Aniskovich.
Continuing Evolution
Jumo Health’s creative roots mean it is ever-evolving. Short-term initiatives include expanding its reach of work in drug discovery to include observational studies and a specific curriculum for RACE Act-specific clinical trials. As it looks deeper into the future, it can see working with payors and further collaboration with community action boards to drive advocacy.
As study protocols, inclusion criteria, and diversity mandates continue to complicate enrollment and retention, Jumo Health has uniquely positioned itself to support this phase of industry growth. It’s history of addressing health literacy and the special circumstances of underrepresented populations means it is aligned with these broader goals in a way that allows it to stand out from competitors.
Raising Awareness for World Sickle Cell Day
Raising Awareness for World Sickle Cell Day
Today, we celebrate World Sickle Cell Day, a chronic condition which affects approximately 100,000 Americans and occurs in 1 out of every 365 Black or African-American births.1 We had the unique privilege of speaking with Jew-E’L Darbone’, the founder and CEO of Bold Plus+, a community based nonprofit organization dedicated to sickle cell awareness, advocacy, and empowerment. Living with sickle cell type SS, Jew-E’L shares her story about managing the condition, the challenges she has faced, and the impact her voice has had on countless others.
“I was four when I first realized I was different. I went to play with my friends in the sprinkler system and the cold water sent my body into one of the worst [sickle cell] crises that I’ve ever had, ” Jew-E’L recalls. “That was really impactful for me at that young age to realize I can’t do what all my friends are doing.”

Sickle Cell Disease Explained
Sickle cell disease (“SCD”) is a group of inherited red blood cell disorders that affect hemoglobin, a protein that delivers oxygen to cells throughout the body; In sickle cell disease, hemoglobin isn’t made correctly. The hemoglobin forms long chains inside the red blood cells making them curved (or, “sickled,” hence the name) and stiff. These cells, then, can not flow smoothly through the bloodstream, and get stuck, which slows down or blocks blood flow to different parts of the body. This can cause problems in the body such as a blockage of blood flow to the spleen, brain, and lungs, and severe pain. A hallmark of this condition is recurrent, acute, and chronic pain that often requires immediate management and emergency hospitalization.2 When these things happen all of a sudden, it’s called, as Jew-E’L mentions above, a sickle cell crisis.
The Challenges of Living with a Chronic Illness
Diagnosed at birth, Jew-E’L describes the challenges she faced as a young child recalling, “it was very, very isolating. I thought I was the only one in the world that had sickle cell disease and I didn’t have any family [members] who could understand or empathize with me. I really didn’t have any friends or anything like that.” Along with the physical burden of managing a chronic illness, it can also have an effect on a person’s mental health. Evidence suggests that depressive and anxiety disorders are much more prevalent among medically ill children and adolescents when compared with the general population.2 In one study of SCD, 40% of the participants met the criteria for depression.2
Jew-E’L notes, “I dealt with a lot of mental health issues. I dealt with a lot of suicidal thoughts and different things like that.” For Jew-E’L, the opportunity to attend sickle cell camp and establish a strong network of peers was a turning point. She explains, “sickle cell camp really saved my life because I found my tribe. I thought, wow, all these kids are dealing with the same issue that I’m dealing with and they’re just as unique as I am. They’re all different, we all deal with different issues, there’s no two cases of sickle cell that are alike. They call us snowflakes because each one of us is totally different. But, we all go through the same struggle.”
Finding Support and Resources
Access to educational resources are not always readily available, especially in traditionally underserved communities. For Jew-E’L, the first educational tool she received about SCD was at camp when she was 11 years old. “I wish that I had more doctors that spoke to me and not so much directed everything towards my mom. I wish they would have prepared me to become my own best advocate. I really feel like I was late to the game, like, I was a late bloomer when it came to taking care of my own health care decisions and using my voice to speak up for myself,” shares Jew-E’L.
When reflecting on what she wants to see for patients with sickle cell disease, Jew-E’L states, “I wish more doctors would approach the whole patient and not just treat a diagnosis. But actually the entire person — from the mental to physical, to dealing with every aspect of the person and not just the diagnosis of sickle cell disease.”
Becoming an Advocate
Finding strength and support from the sickle cell community is part of what drove Jew-E’L to start Bold Plus+. When asked about her biggest support, Jew-E’L explained, “It’s the strength of our community, being able to really trust one another, to find strength in each other, and be inspired by one another.” Jew-E’L’s social media community reaches over 5,000 people, giving people with sickle cell disease the opportunity to connect and share knowledge and resources. While Jew-E’L didn’t have this type of online support network growing up, she is working hard to ensure all patients with sickle cell have this resource moving forward.
So today, we celebrate Jew-E’L and her network of sickle cell warriors who fearlessly share their stories in an effort to help improve the journey and lives of other patients with sickle cell disease.
At Jumo Health, rare diseases, such as sickle cell disease, is an area where we have dedicated considerable energy and effort. To date, we have provided sickle cell disease resources across 27 different countries and in 25 languages. Our mission is to ensure that regardless of age, educational attainment, or socio-economic status, patients and their entire care circle can have access to information they can understand and act upon — whether it be at diagnosis, during treatment regimen, or while participating in a clinical trial. To learn more about our solutions, visit blog.jumohealth.com/solutions. To access our sickle cell resources, click here.
We are so appreciative of Jew-E’Ls time talking with us and her passion to serve the community that we are providing a 20% discount off all of our SCD resources all weekend long!
About Bold Plus+
Bold Plus+ is a community based non profit organization dedicated to sickle cell awareness, advocacy, and empowerment. You can follow them on Facebook and Instagram.
Sources:
- Centers of Disease Control and Prevention. Data & Statistics on Sickle Cell Disease. Accessed June 18, 2020. https://www.cdc.gov/ncbddd/sicklecell/data.html
- Benton, T et al. Anxiety and depression in children and adolescents with sickle cell disease. Curr Psychiatry Rep. 2007. 9(2):114-21.
The Impact of a Cancer Diagnosis on the Patient and the Entire Care Circle

CELEBRATING NATIONAL CANCER SURVIVORS DAY
Today we celebrate National Cancer Survivors Day. As we honor the lives of those who have battled cancer, we recognize the impact a cancer diagnosis has on the entire care circle — in some cases, inspiring those people to make impactful change in the lives of future patients. This piece shares the stories of just two in a sea of individuals who have confronted and beaten cancer.
Jen, an emergency room nurse for 15 years, still remembers vividly the day she was diagnosed with nasopharyngeal carcinoma. “As soon as [the doctor] put the scope in my nose… his entire face changed. And, it was at that moment, I knew I had cancer.” From that moment on, it was a non-stop road to recovery — assembling her care team, Jen worked tirelessly to research her condition, go to her treatment, and deal with its grueling side effects. Jen continued, “I didn’t tell anyone [when I was first diagnosed]. I went straight into nurse mode… I wanted to know all the data. How do we treat it? How do we beat it? I wanted to know the survival rates. I wanted to know everything. I couldn’t get in my feelings about it — I went straight into ‘what do I need to do next’… straight into fight mode.”

Shoby tells a similar story when her brother, Yadev, was diagnosed with Acute Lymphoblastic Leukemia (ALL) when he was one year old. “I still remember the moment the results were being [shared with] my mother. I was just outside of the room playing with the toys provided by the hospital… something didn’t seem right as the doctors had a somber look on their faces. It wasn’t until they had left the room that I went in to find my mum crying in hysterics and explaining it over the phone to my family.” Being nine years old at the time, she had only heard the word cancer in association with the word death, so she “instantly thought of the worst case scenario — that my brother would only have a matter of time to live.”
Shoby’s mother, Jan, noted, “When [the doctors] left the room I hugged my baby so tightly and cried with my tears dripping all over his baby gown. I was so terrified that my child was going to die and I could not do anything about it. I realized at this point that I need[ed] to get a grip and deal with this head on. Coming from a big Asian family who are extremely tight knit, I called my family and my cousins. Within moments, all of them were at the hospital and my family overtook the ward.”
Common Challenges Faced by Families Affected by Cancer
Studies have shown that patients face many challenges while managing a cancer diagnosis, including financial, social, communication, and logistical barriers.1 In one study, researchers found that problems with medical communication and lack of social support affected 50% of the patients.1 Such examples include reluctance to ask questions or share problems with the medical team.1
These barriers were also experienced by both Jen’s and Shoby’s families. Jen describes how her cancer case was unique and required a tremendous amount of her time and self-advocacy. “One of the biggest challenges during treatment was navigating the process. While I was ok because I’m a nurse, other people might not be,” said Jen. “For somebody who doesn’t know anything about health care, I can imagine them getting so lost and so overwhelmed in the system.”
The Need for Educational Resources and Support
Shoby also expressed feeling lost and confused, explaining that her brother’s diagnosis was not explained to her in a way she could understand. “Although I can vaguely remember the doctors trying to explain what was wrong with my brother, my understanding of his cancer was that it was blood related, as it was all too technical for me at the time. I thought that his blood was poisonous. It wasn’t until my aunt Sindy… gave me a book with Medikidz characters explaining Leukemia that I got a better understanding of what the cancer was.” When asked what resources she wished she had when her son was diagnosed, Jan said, “I wish that there were some simple books or pamphlets I could have been given to know about the disease, books to explain to my daughter what her brother was going through and what she should be expecting.”
In addition to the communication and logistical challenges they faced, their stories also highlighted how a cancer diagnosis not only affects the patient, but the entire care circle. Jan expressed the guilt over her son’s diagnosis, “I felt as though I was the cause of his sickness and that my genetics contributed to his disease. I questioned whether I could have done something to avoid this — was it because I heated his milk in the microwave? Was it because I didn’t feed him the right foods? For my daughter [Shoby, who was attending school], I could not focus on her and her homework.” Shoby explained, “I think the biggest challenge for me as a kid during the time was feeling lonely. At the time, I felt as though my brother always got all the attention and I felt left out, I wouldn’t have anyone attend my school plays, or drop me off on my school trips.”
Jen also noted how her cancer required non-stop support from her family — taking her to all her appointments, setting up a fundraiser to help with costs, and having her sister sleep in her room every night to make sure she was okay. “I would have never survived without that support,” Jen said.

The Road Ahead
The stories of Jen, Shoby, and Jan all speak to how a cancer diagnosis marks one’s life, and how the impact can be the catalyst of change. At Jumo Health, many of the team members have a personal medical story that led them to the field of health care. In fact, for two team members, it was a family cancer diagnosis that led them to careers in health care. For Kevin Aniskovich, President and CEO of Jumo Health, he started his career in health care when his mother was diagnosed with cancer. For Sindy Nathan, Area Vice President of Strategic Solutions, it was her family (Shoby, Yadev, and Jan) that inspired her career trajectory — making her want to bridge the communication gap and provide resources that explain medical conditions in a way the entire family could understand. For Shoby, the frequent trips to the hospital during her brother’s treatment and seeing the work the doctors did to save her brother’s life inspired her to give back. She is currently studying to get her Masters in Global Health. “I feel as though it is my way of giving back to the community,” she said.
When asked about what they would recommend to other people affected by cancer, they spoke to the need for support and talking with other people with cancer. As Jen said, “[Patients with cancer] are literally the only people on planet earth that know what you are or are about to go through. Not the doctors, not your friends, not your family, not your therapist, not your coworkers. Other cancer patients — those people will be your best ally.”
So today, we celebrate the lives of those who have been affected by cancer, recognizing their stories of triumph, loss, perseverance, and resilience. Thank you to Jen, Shoby, and Jan who fearlessly shared their stories so that others going through a similar circumstance can know they are not alone.
It’s stories like these that led to the founding of Jumo Health and that keep us striving to provide authentic, relatable resources during the most pressing times. At Jumo Health, we provide age appropriate educational resources for patients and their care circle for use throughout their medical journey. We work to ensure that regardless of age, educational attainment, or socio-economic status, patients and their entire care circle can understand and act upon a medical diagnosis and treatment plan. To learn more about our solutions, visit blog.jumohealth.com/solutions. Find more information about our Oncology resources, here.
Source:
1. Harden S et al. Patients’ barriers to receipt of cancer care, and factors associated with needing more assistance from a patient navigator. J Natl Med Assoc. 2011; 103(8):701–710.
Testing for COVID-19
Want to download this infographic? Click here. For more COVID-19 resources, visit our COVID-19 Resource Center.
For more guidance and resources, check out these helpful resources from the CDC:
A Salute to Nurses
Nurses — their commitment, contributions, compassion, and care — play a critical role in our health care system. With over 4 million registered nurses in the United States, it’s likely that we all have someone in our lives who has answered the call to care for others. In honor of National Nurses Day, we spoke with a few nurses who are close to our hearts here at Jumo Health. We asked them to answer some questions about what led them to nursing and what being a nurse means to them. Here’s what they had to say…
Amanda Altieri
What made you become a nurse?
I wanted to become a nurse after many summers volunteering at a pediatric cancer camp. I felt that the bond between our campers and the nurses that took care of them were so important and I wanted to help people in that same special way. One of my aunts who was a part of the original medical staff at the camp was a major cheerleader in my career path too!
What do you love about being a nurse?
Even though I am not a pediatric nurse which was the driving force for my second degree and career change, what is so amazing about nursing is that there are so many different avenues for one career. I ended up in the Operating Room and am on the cutting edge of technology and innovation in the medical field. I’m responsible for so much and the patients put their trust in our team while they are on the operating room table. During this COVID-19 crisis, I was able to help deliver babies in labor and delivery, and assisted on a C-section for my longest friend from kindergarten — it was amazing!
What are 3 words you would use to describe the work you do?
Transformative, healing, and innovative.
What is a phrase or quote that keeps you going during difficult times?
Have some fire, be unstoppable, be a force of nature.
Kristie Heimerle
What made you become a nurse?
I wanted to help people.
What do you love about being a nurse?
I love taking care of people and their families.
What 3 words would you use to describe the work you do?
Rewarding, compassionate, and challenging.
What is a phrase or quote that keeps you going during difficult times?
When it rains look for rainbows, when it’s dark look for stars.
Kristi Pfohl
What made you become a nurse?
I started in retail management and my three sisters were all nurses. I liked to hear their stories and wanted to make a difference in people’s lives, so I went to night school and got my degree.
What do you love about being a nurse?
I love working with patients and helping people. I also enjoy the flexible hours when raising a family and being able to schedule around my children’s activities.
What are 3 words you would use to describe the work you do?
Challenging, rewarding, and important.
What is a phrase or quote that keeps you going during difficult times?
I always feel that the “difficult times will pass” and the camaraderie with my fellow nurses will pull us through.
Want to learn more about the hospital team? Check out this resource from our team!
A Discussion on Innovation in Clinical Trials
Jumo Health Participates in a Tweet Chat with Experts in the Industry
Today is International Clinical Trials Day. To celebrate, we participated in a tweet chat with our friends at Eli Lilly and other experts in the industry about how innovation is taking hold in clinical trials. The conversation focused on how innovation should be centered on the patient and the importance of practical solutions that solve for the core issues. Read below for some of our thoughts on the topic in 280 characters or less…
Question 1: How would you define “innovation” when it comes to clinical trials?
Thought #1: Innovation is intended to ease a burden or solve a problem. Jumo Health innovates by providing solutions that work the way people live. We do this to ensure mass appeal and broad utilization. Without this, innovation is simply fodder for textbooks.
Thought #2: All innovation in clinical trials should be centered around the patient. While some may think AI, at Jumo Health, we address core issues that prevent optimal outcomes and focus on age appropriate resources to overcome health literacy challenges.
Thought #3: A practical approach to innovation matters because the stats don’t lie. ~85% of all clinical trials experience delays. 50% of adults can’t read above an 8th grade level, and non-compliance hovers ~70%. Innovation should focus on the root cause.
Thought #4: Our definition of innovation is embodied in our product design and through a range of age appropriate offerings from comic books to animations — all of which are intended to allow patients to understand and act upon their health care instructions.
Question 2: What recent innovations have you seen that have created a more patient-centric clinical trial experience?
Thought #1: The paradigm shift of placing patients at the center of product design is finally taking hold in clinical trials. Will this innovation make an impact on increasing health literacy? If so, then achieving optimal outcomes and compliance is right behind.
Thought #2: The informed consent process is broken. We love when organizations take steps to produce materials that augment the med-legal requirements with patient-friendly, engaging, and actionable resources.
Thought #3: We applaud organizations that take a patient-first approach. Our friends at Eli Lilly used mixed media resources, tailored for each age cohort, to break down complex medical information and make the unknown less scary. Check it out here.
Question 3: CISCRP’s 2019 Perceptions and Insights Study found that technology is playing an increasingly larger role in clinical trials. What technology do you think has been the most impactful for the patient experience?
Thought #1: Even though the study was fielded before the COVID-19 pandemic, the future role of tech in patient-centered health care is tricky due to HIPAA and GDPR, and further complicated by those who are traditionally underserved without access to certain tech.
Thought #2: ePRO, eCOA, and telemedicine solutions have taken positive steps in the space to help increase access and ease the day to day. The platforms also require best in class educational resources to become a stronghold.
Thought #3: Virtual reality is just starting to play a role. Long study visits often increase the patient’s fear and anxiety. The immersive experiences provided help calm and distract participants while supporting overall well-being.
Question 4: How can innovation lower barriers to participation in clinical trials?
Thought #1: Innovation is not a panacea. Fundamental educational deficiencies plague the health care industry. To push clinical trials forward using tech such as IBM Watson, we need to ensure patient-friendly materials are at the forefront of the experience.
Thought #2: Allow for a patient-friendly informed consent experience. Period. As study protocols become more complex, the onus is on us to ensure patients and caregivers can understand what is being asked of them. This upfront step will reduce dropout.
Thought #3: Informed consents are typically written at an 11th grade reading level; yet 50% of adults can’t read above an 8th grade reading level. 35% of people who dropped out of a clinical trial found the informed consent difficult to understand.
Thought #4: Businesses that reach out to the community physicians to ensure those traditionally underserved can benefit from novel approaches to treatment such as Elligo Health.
Thought #5: Mixed media resources that are available in the community will ensure that every patient, regardless of educational attainment or socio-economic status, can understand, manage, and own their own health.
Question 5: What is an innovation that you’re hoping to see in clinical trials in the near future?
Thought #1: Enterprise tech such as IBM Watson shows promise but in order to more effectively impact patient care and outcomes, we are bullish on basic building blocks such as data analytics to identify key inflection points and customer service platforms.
Thought #2: Wearable devices that can help track a participant’s progress, send reminders and positive notes, and allow for easier communication between participants and site staff.
Thought #3: Customer service platforms! Treat a participant like a consumer. Innovation doesn’t need to re-invent the wheel — it often just needs to apply decades of learnings to health care.
Thought #4: Actionable patient resources! We are committed to solving the fundamental challenge that plagues the point of care by delivering patient-friendly, evidence-based, and peer reviewed resources.
Thought #5: A greater focus on pediatric trials both related to the RACE Act and among all disease types.
Thought #6: Expanded health literacy! We see a future where the statistic that 90% of the population are health literate — acting upon their physicians instructions to meet their end goals.
Thought #7: Solutions that bridge relationships between patients, the site staff, and the larger clinical trial community. Patients want a sense of connection and to be appreciated for their participation.
We Are Jumo Health: Columba Quigley, MD
An Interview with Editor in Chief, Columba Quigley, MD
Great companies are made up of great people. Here at Jumo Health, our most valuable resource is our team. We are a collective of medical folk, product people, designers, and storytellers that share a common goal to change health care today. Through our We Are Jumo Health series, we will introduce you to the dedicated people who are the heartbeat of Jumo Health.
This month, we’d like you to meet Columba Quigley, MD, Editor in Chief of Jumo Health. Columba has been a member of our team for nine years. Her expertise and wisdom has proven invaluable not only to the work we create, but also to her colleagues who seek her guidance. Meet Columba!

What led you to Jumo Health?
My background is in medicine and I worked as a hospital-based physician for many years. Over that time, I became increasingly interested in the language of medicine, in how we communicate stories of illness, and also the gap that seems to exist between “medical jargon” and a patient’s understanding. Initially, I studied the language of illness from an academic perspective, becoming a humanities scholar in the field. One of the areas I was specifically interested in was the world of graphic medicine — the power of that specific visual and verbal format to uniquely communicate stories of illness.
What is your favorite part about working at Jumo Health?
What we achieve: making the confusing less so for a potentially vulnerable audience; who I work with: an amazing team that is both creative and compassionate, and fueled by a similar passion.

What motivates you?
The belief that I might be helping those who are ill and at the same time confused and anxious about their medical condition and what they might be facing. Also, I believe that “knowledge is power” and if we can help people understand their illness and why they need to comply with prescribed treatment regimes, then their clinical outcome may actually significantly improve.
What is your proudest achievement at Jumo Health?
Increased focus on health literacy. By working to ensure that our resources are truly literacy sensitive and truly accessible to and understandable by our target audience.
What are you most excited about for the future of Jumo Health?
Having witnessed the company achieve so much over the past years, I am really excited about its potential to realize even more achievements in the health care space.
If you could do any other job for one day, what would it be and why?
Be a concert pianist. Because it might transport even one audience member to a place of (albeit transient) sublime joy.
What is the most important thing you’ve learned in the last five years?
Humility.
Finish this sentence, I am happiest when…
I happen upon random and unexpected moments of joy.
If you want to learn more about Columba, you can connect with her on LinkedIn. Want to work for Jumo Health? Check out our career opportunities!
What is a Virus? Our Resource Helps Explain
During this trying time for our global community, it’s safe to say one word on everyone’s mind is “virus.” But, where to turn for safe, reliable information that you don’t need a white coat to understand can sometimes be difficult.
At Jumo Health, we create highly visual resources based on the latest medical research using storytelling to explain difficult medical concepts. With such an influx of information being relayed from all directions surrounding the coronavirus and COVID-19, it can be helpful to take a step back and start at the beginning. We have a number of resources relevant to the current pandemic.
To start with, our title on infections addresses a key question that may not be the first to come to mind, but is particularly relevant: what is a virus?
It can be difficult answering even this simple question for kids. Understanding Infections addresses this difficulty by creating an engaging adventure story around the medical facts, in a language and style that engages and entertains while simultaneously educating. As the fictional “Mediland” becomes “invaded” by germs (a narrative that resonates with what the world is currently experiencing) two of the resident superheros (the “Medikidz”), Axon and Abacus, explain what infections are and the different types of causative germs, including the type on everyone’s minds.
As the story continues, the Medikidz proceed to explain various types of infections, from the common cold to more serious conditions, such as meningitis. How infections can be treated and prevented is also explained.
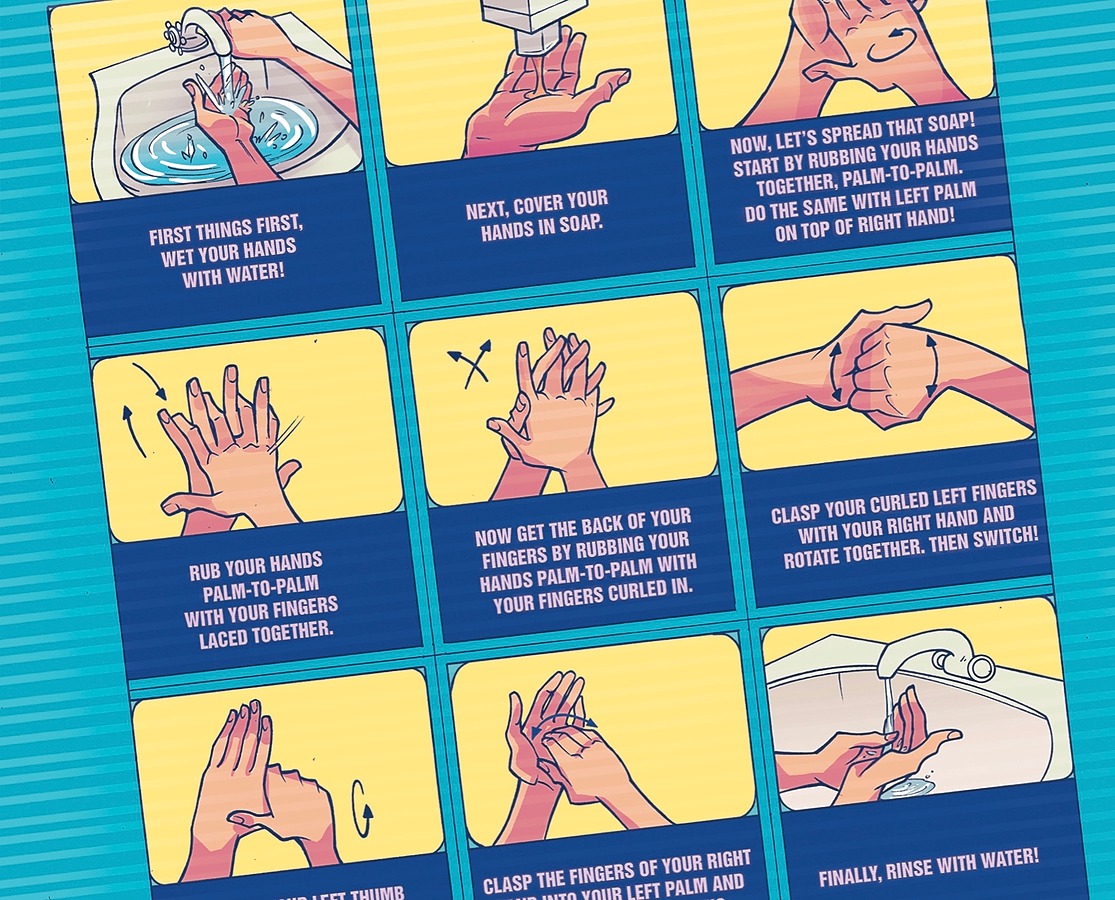
The book ends with the most important message of all— the best way to prevent infections is through regular and thorough handwashing. The current pandemic has highlighted the fact that how we have been washing our hands to date generally falls well short in terms of preventing the spread of disease. Understanding Infections includes a full page visual step-by-step guide on how we should be doing this critical infection prevention measure.
Understanding Infections answers simple questions that our young audience ask and can relate to. Arming oneself with facts and thereby influencing health behavior is very much at the core of the Jumo Health ethos.
Read our Understanding Infections book here!